Portal
-
Trump to Stop Deportations If…
Monday, November 03, 2025President Donald Trump invited the Dodgers to the White House. Many of their fans feared that the team, by accepting, would humiliate themselves and betray the team’s large Latino, Asian and African-American fan base. Dodgers controlling owner Mark Walter, along with co-owner Magic Johnson, have proposed a solution. Trump has promised that if he can keep the championship trophy, the Commissioner’s Trophy, he will end all seizures and deportations of immigrants. read more -

Harm to Iraqi and Afghan Civilians from U.S. Military Burn Pits Largely Ignored by Mainstream Media
Tuesday, October 20, 2015"The vast majority of news stories made no mention that Iraqi and Afghan civilians might also have been harmed by the U.S. military’s burning of waste,” wrote Bonds. “When journalists describe the pollution itself, how it billowed over military bases and covered living quarters with ash and soot, such accounts never mention that this pollution would not have stopped at the cement barricades...at base boundaries, but must have also settled over civilians’ homes and the surrounding landscapes.” read more -

Titles of Government Reports you’re Not Allowed to See are Published by GAO…Except for Titles You’re Not Allowed to See
Tuesday, October 20, 2015The Government Accountability Office (GAO) “quietly published” a list of titles of its restricted reports that have not been publicly released because they contain “classified information or controlled unclassified information.” GAO officials said the list was published in an effort to inform lawmakers, federal agencies and the public about those reports. The list goes back only as far as September 30, 2014, and it does “not cite titles that are themselves classified,” Steven Aftergood wrote. read more -

Gay U.S. Ambassador to Denmark Marries his Partner
Tuesday, October 20, 2015Gifford said coming out as a teenager was challenging, but that his life has changed completely since then. “I came out when I was 18... It was a huge struggle. I had no gay role models... I was riddled with self-hatred and self-doubt, and a lack of any understanding of what my life would be like in the future. There were many, many days when you didn’t want to wake up the next morning, but you can’t even imagine those days now—they seem like another lifetime.” read more -

National Weather Service Leadership Clashing with its Employees about Non-Disclosure
Tuesday, October 20, 2015The union representing workers at the National Weather Service (NWS) has filed a legal complaint against the agency’s recent introduction of employee nondisclosure agreements, claiming managers are trying to “gag” staff from talking about internal issues. The NWS’ nondisclosure orders forbid disclosing information about activities related to workforce planning, settlement of grievance disputes and the collective bargaining process. read more -

Most Victims of U.S. Drone Targeted Killing Program Aren’t the Targets
Monday, October 19, 2015During one year, U.S. drone strikes killed more than 200 people—but only 35 of them were the intended targets. “These eye-opening disclosures make a mockery of U.S. government claims that its lethal force operations are based on reliable intelligence and limited to lawful targets,” said ACLU. “The government often claims successes that are really tragic losses." Said the whistleblower: "Assigning...death sentences without notice, on a worldwide battlefield...was, from the [beginning], wrong.” read more -
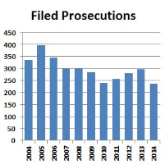
Prosecution of Corporations Drops under Obama
Monday, October 19, 2015Criminal prosecutions of corporations declined by nearly 30% from 2004 to 2014. The hands-off approach by the Obama administration began at the end of the Bush years, when Deputy Attorney General Mark Filip told federal prosecutors in 2008 “take into account the possible substantial consequences to a corporation’s employees, investors, pensioners and customers” when thinking of going after a company. There were 21% fewer corporate prosecutions in the five years after the Filip memo. read more -

Climate Change Doubters—Particularly Among Republicans—Hit Record Low
Monday, October 19, 2015The greater numbers of people now experiencing the effects of climate change have contributed to the switch. “The drought issue is affecting big regions of the country,” said Rabe. “Drought is not just a narrow, localized issue now.” Fifty-six percent of Republicans support the evidence behind global warming. That’s the highest that number has been since 2008, just as the GOP establishment began to attack President Barack Obama and his policies, including climate change mitigation. read more -

DuPont Found Liable for Woman’s Kidney Cancer
Monday, October 19, 2015It was revealed at trial that DuPont knew of the potential toxicity of C8 since the 1950s. Even DuPont’s defense witnesses presented damning testimony. One employee admitted he had 400 parts per billion of C8 in his blood, about 100 times the national average. “I knew there were a lot of other people who had much higher levels, and so I didn’t think mine was anything to worry about,” he said. “Everything is toxic.” Another ex-employee said he had a possibly cancerous spot on his kidney. read more -

U.S. Pulls Plug on New Arctic Oil Drilling Leases
Monday, October 19, 2015The announcement was made the month after Shell gave up on its exploration efforts in the Alaskan Arctic after spending seven years and $7 billion on the efforts. The decision also came as the price of oil stabilized around the $50-per-barrel mark with a glut of the product in the U.S. For now, it means an estimated 13% of the world’s unexplored oil reserves will remain in the ground, perhaps slowing the effects of climate change on the fragile Alaskan landscape. read more -

Okinawa Governor Halts Construction of U.S. Marine Corps Air Base
Sunday, October 18, 2015Onaga’s action is being fought by Japan’s central government, but a revocation would likely come with a political cost to the prime minister. Already, many of Abe’s national security actions have triggered widespread opposition. Okinawans have been eager to get U.S. forces off their island. Two notorious rapes committed by U.S. personnel, one a gang-rape of a 12-year-old girl in 1995, have caused considerable antipathy toward Americans, read more -
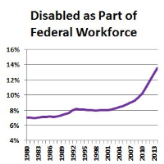
Record Number of Disabled Americans Work for U.S. Government
Sunday, October 18, 2015As of 2014, 248,608 federal employees were classified as disabled, including veterans with 30% or more disability. That’s 13.6% of the federal workforce, an increase of 0.8% over 2013’s numbers. It’s also the largest number and highest percentage of disabled federal employees since 1980. Obama signed an executive order in 2010 requiring the hiring of 100,000 disabled workers by the federal government within five years. OPM reports that agencies are “on track” to meet Obama’s requirement. read more -

Acting Secretary of Education: Who Is John B. King?
Sunday, October 18, 2015King has said his idea of an ideal school is one with a rigorous curriculum, excellent teachers, a longer school day and a longer school year. He also wants a focus on data to give teachers a picture of how their students are performing. Since Republicans had dragged their feet in approving even the most routine nominations until recently, Obama sidestepped the process when he could. After Duncan steps down at the end of the year, King will serve as secretary in an acting role. read more -

U.S. Ambassador to Nicaragua: Who Is Laura Dogu?
Sunday, October 18, 2015In 2007, she was made Consul General in Ciudad Juarez, Mexico. Part of her duties there involved dealing with American victims of the drug war that raged in the border region. While serving in the State Dept, Dogu has also put her business acumen to good use. She became a frequent poster on the personal financial planning forum Bogleheads.org and co-authored a book, The Bogleheads’ Guide to Retirement Planning. In 2012, Money magazine named her a champion of small investors. read more -

Administrator of the Pipeline and Hazardous Materials Safety Administration: Who Is Marie Therese Dominguez?
Sunday, October 18, 2015Dominguez has had extensive government service in Democratic administrations There has been concern among some that is unqualified for the position as she has little experience dealing with pipeline issues — she is basically an unknown in the industry. Her backers counter that she’s a good administrator and has good connections to the White House, which will help in implementing regulations aimed at keeping America’s pipelines from rupturing and trains carrying crude oil from derailing. read more -

Miami and New Orleans among 400 U.S. Cities said to be Doomed by Rising Sea Levels
Saturday, October 17, 2015In New Orleans, 98% of populated land would be below sea level. “So it’s really just a question of building suitable defenses or eventually abandoning the city,” said study author Benjamin Straus. Miami is just one of many cities in Florida at risk, with at least 40% of the people who face having to move because of climate change. The 1.5 million people at risk in New York City and the 100,000 in Philadelphia might not be displaced if carbon emissions are drastically cut. read more -

FDA Committee Ties to Drug Industry Underlie Lax Oversight of Controversial Blood-Thinner
Saturday, October 17, 2015Texas cardiologist Darren McGuire was on the FDA committee that approved Pradaxa. He later disclosed that he received “personal fees from [Pradaxa manufacturer] Boehringer Ingelheim,” paying him between $75,000 and $134,994 over a three-year period. FDA advisory committee member Sanjay Kaul, a medical professor at UCLA and cardiologist at Cedars-Sinai Medical Center, also received payments from Boehringer Ingelheim in 2013 totaling more than $21,000, and $75,000 a year later. read more




