Are FDA Opioid Warning Labels Too Little Too Late?
 (photo: Joe Raedle, Getty Images)
(photo: Joe Raedle, Getty Images)
By Matthew Perrone, AP Health Writer
WASHINGTON (AP) — Federal health regulators will add their strongest warning labels to the most widely prescribed painkillers, part of a multi-pronged government campaign to stem an epidemic of abuse and death tied to drugs like Vicodin and Percocet.
The Food and Drug Administration announced Tuesday plans to add a boxed warning — the most serious type — to all immediate-release opioid painkillers, including some 175 branded and generic drugs.
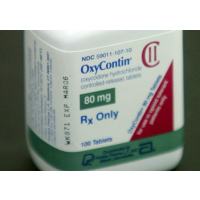
Those medications, which often combine oxycodone with lower-grade medications, are among the most commonly used drugs in the U.S. and account for 90 percent of all opioid painkillers prescribed. Roughly three years ago the FDA added similar warnings to long-acting opioid drugs like OxyContin, which slowly release their doses over 12 hours or more. Now both immediate and extended-release formulations will highlight the risks of addiction, abuse, overdose and death.
The long-awaited changes come as federal and state officials struggle to curb a wave of overdoses fueled by the overprescribing of medications and a steady supply of cheap heroin.
"We're at a time when the unfathomable tragedies resulting from addiction, overdose and death have become one of the most urgent and devastating public health crises facing our country," FDA Commissioner Dr. Robert Califf said on a call with reporters. "I can't stress enough how critical it is for prescribers to have the most current information."
But lawmakers from states that have been ravaged by opioid addiction said such labeling changes have "done little" to help their communities.
"Unfortunately, it has taken FDA far too long to address the grave risks of these drugs that have claimed the lives of thousands this year alone," said Sen. Edward Markey, D-Mass.
Opioids are a class of powerful and highly addictive drugs that include both prescription drugs like codeine and hydrocodone, as well as illegal narcotics, like heroin.
Deaths linked to misuse and abuse of prescription opioids climbed to 19,000 in 2014, the highest figure on record, according to the Centers for Disease Control and Prevention. Heroin and opioid painkillers combined caused 28,650 fatal overdoses.
Doctors are not required to follow the FDA's instructions on drug labels, though they are often used as prescribing guidelines by hospitals, medical groups and insurers.
Critics of the FDA, including Physicians for Responsible Opioid Prescribing, called on the agency to add such warnings years ago.
"The main driver of our opioid epidemic is addiction, and the immediate-release products are just as addictive," said the group's founder, Dr. Andrew Kolodny, an addiction therapist with Phoenix House, a network of rehabilitation clinics.
The new FDA label will specify that the drugs like Percocet should only be used when other medications and alternative therapies cannot control patients' pain.
"This new indication, once finalized, will remind prescribers that immediate-release opioids are also powerful drugs with important safety concerns," said Dr. Doug Throckmorton, a deputy director in the FDA's drug center.
Throckmorton said the agency's 2013 labeling change focused on long-acting drugs like OxyContin because they represented a "disproportionate risk" to patients, since they contain larger opioid levels.
Government officials have tried a variety of approaches to tackling painkiller abuse in recent years. The FDA previously restricted combination pills like Vicodin to limit refills and who can prescribe them. States like Florida and New York have cracked down on "pill mills" using databases to monitor what doctors are prescribing.
The Obama administration has asked Congress to provide $1.1 billion to combat opioid addiction. On Tuesday, the White House sent letters to all 50 governors outlining steps for reducing opioid over-prescribing and enhancing addiction treatment.
The administration's drug czar praised the move by the FDA and Califf, who was confirmed to lead the agency last month.
"I think we're very heartened by the new director of the FDA and his enhanced focus on opioid issues," said Michael Botticelli, National Drug Control Policy Director. "This is an all-of-government effort and clearly the FDA has a key role to play."
In addition to the boxed warning, the FDA said it would add new information about the risks of opioid use for pregnant women and their newborns as well as drug interactions with antidepressants and other medications.
The FDA announcement comes less than a week after the CDC released the first-ever national prescribing guidelines for using opioids. The agency said primary care doctors should only turn to opioids after considering physical therapy, over-the-counter medications, counseling and other methods for treating chronic pain.
To Learn More:
CDC Urges Doctors to Render Prescription Painkillers a Last Resort (by Matthew Perrone, Associated Press)
New FDA Painkiller Labeling Rules Seen as Good PR but Bad Medicine (by Noel Brinkerhoff and Danny Biederman, AllGov)
FDA Panel Advises Limiting Prescription Refills for Addictive Painkillers (by Matt Bewig, AllGov)
- Top Stories
- Unusual News
- Where is the Money Going?
- Controversies
- U.S. and the World
- Appointments and Resignations
- Latest News
- Trump Orders ICE and Border Patrol to Kill More Protestors
- Trump Renames National Football League National Trump League
- Trump to Stop Deportations If…
- Trump Denounces World Series
- What If China Invaded the United States?






Comments